Comments
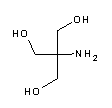
Tris(hydroxymethyl)aminomethane (abbreviated TRIS or THAM), also known as tromethamine, trometamol (INN, International Nonproprietary Name) and TRIS buffer. Chemically, it is a primary amine with three alcoholic hydroxyl groups.
Synthesis
First, three moles of formaldehyde are added to nitromethane in an aldol reaction to form tris(hydroxymethyl)nitromethane. Subsequently, the nitro group is reduced to the amino group.
Chemical properties
Tris(hydroxymethyl)aminomethane is used for biochemical, molecular biological, microbiological and pharmaceutical purposes as a buffer substance. At a pKa 8.2 (at 20 °C), TRIS has a good buffering capacity between pH 7.2-9.0, but it shows a relatively strong temperature dependence of the acid constant (ΔpKa = -0.031 K-1).The pH increases when the solution is cooled or decreases when the solution is heated.
Content determination
The content of tris(hydroxymethyl)aminomethane can be determined as a weak base in aqueous medium using methyl red as an indicator against hydrochloric acid. As an ethanolamine derivative, TRIS can be detected by the Chen-Kao reaction. In this reaction, the substance is mixed with sodium hydroxide solution and copper(II) sulfate (CuSO4) (blue-violet coloration).
Use
Buffer substance in biochemistry
Tris is commonly used as a buffer substance in biochemistry, e.g. in the TE buffer for DNA purification or in electrophoresis buffers such as for the separation of proteins by SDS-PAGE or the separation of nucleic acids by agarose gel electrophoresis (TAE buffer, TBE buffer). Furthermore, tris(hydroxymethyl)aminomethane is used for buffers to solubilize proteins, e.g. in TBS buffer and TBST buffer. Since the compound has a reactive primary amino group, the buffer is not suitable for some chemical applications, which is why Good buffers or inorganic buffers are then used.
Excipient in pharmaceuticals
Trometamol is used in various pharmaceutical dosage forms such as injection and infusion solutions, eye drops, creams and gels as an excipient for stabilization. It has an alkalizing and buffering effect.
Drug substance/buffer substance in medicine
In the form of its hydrochloride, tris(hydroxymethyl)aminomethane is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of poisoning with weakly acidic substances such as barbiturates. It is administered intravenously. Since Tris buffer has a respiratory depressant effect, its use is contraindicated in patients with respiratory insufficiency who are still breathing spontaneously. As an organic base, tris(hydroxymethyl)aminomethane forms salts with mineral acids. Carbon dioxide (CO2) dissolved in the blood can be neutralized in this way. At the physiological pH of 7.4, approx. 70 % of the tris(hydroxymethyl)aminomethane in the blood plasma is in the ionized (protonated) form. Non-ionized trometamol penetrates cell membranes and is also effective intracellularly as a buffer; this causes potassium shifts from the intra- to the extracellular space. Hyperkalemia and secondary hypokalemia may occur initially. The hydrochloride of the compound is available as an infusion solution or infusion solution concentrate.
Tris is the most commonly used buffer in biological research. One of the most important applications is the use as electrophoresis buffer such as TBE (see A1417 and A0972) or TAE (see A1691) for polyacrylamide and agarose gel electrophoresis, respectively. Tris should not be used at pH values below pH 7.2 or above pH 9.0. The pH value of a Tris buffer strongly depends on the temperature. Therefore, Tris buffers should be prepared at the temperature where it is used. Besides, dilution of concentrated Tris buffers will result in a decrease in the pH value of 0.1 pH units per tenfold dilution. Please take into account when preparing RNase-free reagents by DEPC-treatment that Tris will inactivate DEPC. In addition, Tris may form Schiff bases with aldehydes and ketones.
FAQs
What is the CAS number of Tris?
The CAS number of Tris is 77-86-1.CAS Tris?
The CAS number of Tris is 77-86-1.CAS 77-86-1?
The CAS number 77-86-1 is assigned to Tris. What is Tris?
Tris is the abbreviated name for the organic compound tris(hydroxymethyl)aminomethane, formula (HOCH2)3CNH2, also known as Tris base, THAM, tromethamine, tromethanol or 2-amino-2-hydroxymethyl-1,3-propanediol. It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06 at 25 °C, with effective buffering capacity in a pH range between 7 and 9. What is Tris base?
Tris base (also known as Tris, tromethamine or THAM) is the abbreviated name for the organic compound tris(hydroxymethyl)aminomethane, formula (HOCH2)3CNH2. It is called Tris base (it is the basic, non-ionized form of the amine) to differentiate it from the acid form, the hydrochloride or hydrochloride (Tris HCl). It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. What is Tris hydroxymethyl aminomethane?
Tris(hydroxymethyl)aminomethane is an organic compound, formula (HOCH2)3CNH2, also known as Tris, Tris base, THAM, tromethamine, tromethanol or 2-amino-2-hydroxymethyl-1,3-propanediol. It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. What is tris(hydroxymethyl)aminomethane?
Tris(hydroxymethyl)aminomethane is an organic compound, formula (HOCH2)3CNH2, also known as Tris, Tris base, THAM, tromethamine, tromethanol or 2-amino-2-hydroxymethyl-1,3-propanediol. It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. What is tromethamine?
Tromethamine is an organic compound, formula (HOCH2)3CNH2, also known as tris(hydroxymethyl)aminomethane, Tris, Tris base, THAM, tromethanol or 2-amino-2-hydroxymethyl-1,3-propanediol. It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. What is tromethamol?
Tromethanol is an organic compound, formula (HOCH2)3CNH2, also known as tris(hydroxymethyl)aminomethane, Tris, Tris base, THAM, tromethamine or 2-amino-2-hydroxymethyl-1,3-propanediol. It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. What is THAM?
THAM is one of the abbreviations for the organic compound tris(hydroxymethyl)aminomethane, formula (HOCH2)3CNH2, also known as Tris, Tris base, tromethamine, tromethanol or 2-amino-2-hydroxymethyl-1,3-propanediol. It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. What is 2-amino-2-hydroxymethyl-1,3-propanediol?
2-Amino-2-hydroxymethyl-1,3-propanediol is an organic compound, formula (HOCH2)3CNH2, also known as tris(hydroxymethyl)aminomethane, Tris, Tris base, THAM, tromethamine or tromethanol. It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. What does Tris mean?
Tris is the abbreviated name for the organic compound tris(hydroxymethyl)aminomethane, formula (HOCH2)3CNH2, also known as Tris base, THAM, tromethamine, tromethanol or 2-amino-2-hydroxymethyl-1,3-propanediol. It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. What does Tris base mean?
Tris base (also known as Tris, tromethamine or THAM) is the abbreviated name for the organic compound tris(hydroxymethyl)aminomethane, formula (HOCH2)3CNH2. It is called Tris base (it is the basic, non-ionized form of the amine) to differentiate it from the acid form, the hydrochloride or hydrochloride (Tris HCl). It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. What does THAM mean?
THAM is one of the abbreviations for the organic compound tris(hydroxymethyl)aminomethane, formula (HOCH2)3CNH2, also known as Tris, Tris base, tromethamine, tromethanol or 2-amino-2-hydroxymethyl-1,3-propanediol. It is widely used in biochemistry and molecular biology, in particular to prepare buffer solutions (e.g. Tris-HCl, Tris-Gly, TAE and TBE buffers). Chemically, it is a primary amine with three alcoholic hydroxyl groups, has a pKa of 8.06, with effective buffering capacity in a pH range between 7 and 9. How is Tris used?
Tris is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, drug stability and biological tolerability of the formulation. For example, Tris (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. How is Tris base used?
Tris base is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, Tris (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. How is Tris hydroxymethyl aminomethane used?
Tris(hydroxymethyl)aminomethane is used for biochemical, microbiological, pharmaceutical and molecular biological purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tris(hydroxymethyl)aminomethane (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. How is tris(hydroxymethyl)aminomethane used?
Tris(hydroxymethyl)aminomethane is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tris(hydroxymethyl)aminomethane (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. How is tromethamol used?
Trometamol is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tromethanol is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. How is tromethamine used?
Tromethamine is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tromethamine (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. How is THAM used?
THAM is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, THAM (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. How is 2-amino-2-hydroxymethyl-1,3-propanediol used?
2-Amino-2-hydroxymethyl-1,3-propanediol is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, 2-amino-2-hydroxymethyl-1,3-propanediol (Tris, tromethamine or tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In its hydrochloride form, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What is Tris used for?
Tris is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, drug stability and biological tolerability of the formulation. For example, Tris (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What is Tris base used for?
Tris base is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, Tris (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What is Tris hydroxymethyl aminomethane used for?
Tris(hydroxymethyl)aminomethane is used for biochemical, microbiological, pharmaceutical and molecular biological purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tris(hydroxymethyl)aminomethane (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What is tris(hydroxymethyl)aminomethane used for?
Tris(hydroxymethyl)aminomethane is used for biochemical, microbiological, pharmaceutical and molecular biological purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tris(hydroxymethyl)aminomethane (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What is tromethamol used for?
Trometamol is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tromethanol is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What is tromethamine used for?
Tromethamine is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tromethamine (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What is THAM used for?
THAM is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, THAM (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What is 2-amino-2-hydroxymethyl-1,3-propanediol used for?
2-Amino-2-hydroxymethyl-1,3-propanediol is used for biochemical, microbiological, pharmaceutical and molecular biological purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, 2-amino-2-hydroxymethyl-1,3-propanediol (Tris, THAM or tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What are the applications of Tris?
Tris is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance. What are the applications of Tris base?
Tris base is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, drug stability and biological tolerability of the formulation. For example, Tris (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In its hydrochloride form, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What are the applications of Tris hydroxymethyl aminomethane?
Tris(hydroxymethyl)aminomethane is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tris(hydroxymethyl)aminomethane (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates.What are the applications of tris(hydroxymethyl)aminomethane?
Tris(hydroxymethyl)aminomethane is used for biochemical, microbiological, pharmaceutical and molecular biological purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tris(hydroxymethyl)aminomethane (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What are the applications of tromethamol?
Trometamol is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tromethanol is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What are the applications of tromethamine?
Thromethamine is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, tromethamine (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates. What are the applications of THAM?
THAM is used for biochemical, microbiological, pharmaceutical and molecular biology purposes as a buffer substance or as a component of buffer solutions. For example, in injection and infusion solutions, vaccines, eye drops, creams and gels as an excipient to improve their stability. It has an alkalinizing and pH-regulating effect, i.e. it acts as a pH buffer, regulating and counteracting small changes in the pH of pharmacological solutions. The pH is a crucial parameter in drugs prepared in aqueous liquid form, because it has an impact on the solubility and activity of the molecule, the stability of the drug and the biological tolerability of the formulation. For example, THAM (tromethanol) is a component of some vaccines against COVID-19. It is also used in cosmetics and personal care products, either to adjust the pH of preparations or as an emulsifying, thickening and wetting agent. In the form of its hydrochloride, Tris is used as a drug for the treatment of metabolic acidosis, and also for the alkalinization of urine in cases of intoxication with weakly acidic substances such as barbiturates.
Literature
(1) Good, N.E. et al. (1966) Biochemistry 5, 467-477. Synthesis and characterization of various 'H+ buffers' for biological research. (2) Good, N.E. & Izawa, S. (1972) Methods Enzymol. 24, 53-68. Further discussion on the selection of buffers in biological research, here with emphasis on their suitability for the study of photosynthetic processes. (3) Ogden, R.C. & Adams, D.A. (1987) Methods Enzymol. 152, 61-87. Overview of gel electrophoresis of DNA and RNA with recipes for buffers and solutions and performance of gel electrophoresis.