In Cell Culture it is important to work clean as contaminations are very frustrating for the scientist and in the end also very expensive. ITW Reagents offers a variety of products for prevention, detection and fighting against contamination.
Mycoplasma in Cell Culture
Contamination of cell lines with mycoplasma is a widespread and often underestimated problem. Although visible signs like turbidity or pH-changes are rare, mycoplasma infections can cause a number of cellular alterations, ranging from a decelerated growth rate and altered immunological properties to metabolic and morphologic changes. The consequences of mycoplasma contaminations are serious, with regard to scientific methods like any kind of cell-based assay as well as in terms of health risks for humans and animals.
In this page you will learn more about the Detection of mycoplasma species, the Treatment of contaminated cell cultures and effective Prevention of new infections. We also provide a broad background for an improved understanding of reasons for and consequences of mycoplasma contaminations.
Detection of mycoplasma species

How can I identify a mycoplasma contamination?
The most sensitive method to detect mycoplasma is the direct culture method in suitable broth and agar media to obtain visible colonies. Theoretically, a single CFU (colony forming unit) per sample volume is detectable. Unfortunately this method is also the most time consuming one (up to 28 days; due to the slow growth of mycoplasma species), and it requires experienced personnel conducting the experiments under controlled environmental conditions. Even if the difficult procedure (to start with the complex medium composition often requiring non-standard adjustments for individual species up to analysis of the results) is properly conducted, the method is not 100% effective since some fastidious strains may not grow in pure culture. Therefore, an indirect detection method should be performed in addition.
The most sensitive indirect mycoplasma tests are based on DNA fluorochrome staining (e.g. using DAPI, A1001 ) and PCR. Even if the detection limit of these methods is lower than for the direct culture method, they are absolutely sufficient for routine testings. Commonly, mycoplasma-contaminated cell cultures show high densities of mycoplasma (up to 107 - 108 CFU/ml) that are well suitable for the detection limits of these methods. In contrast to the PCR alternative, the traditional fluorescence staining method requires more time and experience. In addition, the DNA-binding fluorescent stain is cancerous and needs to be handled carefully. Hence, for routine mycoplasma screenings, PCR analysis is recommended. This method is sensitive (depending on the kit almost as sensitive as the direct culture method), very fast (results are obtained within hours) and detects cultivable as well as non-cultivable mycoplasma species. Furthermore, at least with commercially available kits, this method is very easy to perform and does not require a specific expertise.
PanReac AppliChem’s PCR Mycoplasma Test Kits enable identification of mycoplasma-contaminated cell cultures - fast and effective! The PCR technique allows highly sensitive detection of both, cultivable and non-cultivable mycoplasma species. Reproducible results are provided within hours, making PCR the method of choice for frequent routine testings.
| Prod. No. | Description | Features |
| A3744 | PCR Mycoplasma Test Kit | Ready-to-use (Taq Polymerase included!); contains a positive control DNA. |
| A8994 | PCR Mycoplasma Test Kit II | Highest sensitivity of < 10 CFU/ml; according to Ph. Eur. (section 2.6.7). Besides a positive control DNA (non-infectious!), this kit provides an internal control DNA to visualize potential PCR inhibitions. The DNA Polymerase is not included, we recommend a Hotstart Taq Polymerase, e.g. SuperHot Taq DNA Polymerase (A5231) |
PCR Mycoplasma Test Kits: How do they work?!
The principle is simple: A mycoplasma-specific DNA-fragment is amplified exclusively by PCR, using a specific primer set. Only if mycoplasma DNA is present in the sample, a mycoplasma-specific PCR product of defined size (270 bp) is obtained – detectable on an agarose gel, or, in case of the qPCR Test Kit, using the FAM detection channel. The primer set binds selectively to the highly conserved 16S rRNA operon coding region of the mycoplasma genome, not detecting any other bacterial or eukaryotic DNA. The detection range includes the most frequently occurring contaminants M. fermentans, M. arginini, M. orale, M. hyorhinis, M. salivarium, M. hominis, but also less prevalent mycoplasma species, such as M. pneumoniae, Acholeplasma laidlawii, M. synoviae, M. pulmonis, M. bovis - to name just a few.
A new generation of PCR Mycoplasma Test Kits
Besides a Positive Control DNA, the new generation Kit, PCR Mycoplasma Test Kit II A8994 offers an optional Internal Control DNA as inhibition marker. For this purpose, the Internal Control DNA should be added to the master mix to visualize incomplete or inhibited PCR runs. Furthermore, the kits contain a lyophilized Primer/Nucleotide Mix, a buffer solution and PCR grade water. For optimal evaluation of the results, it is highly recommended to always include a negative and a positive control!
What will I get?! How will my results look like?
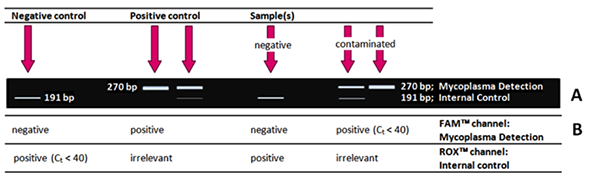
If your PCR was successful, you will typically obtain the following results for negative control, positive control, negative and positiv (contaminated) sample after Standard PCR (Test Kit II, A8994) and evaluation via agarose gel electrophoresis.
Key Factors
- Avoid repeated freezing and thawing. After reconstitution, the Primer/Nucleotide Mix, the Positive Control DNA, and the Internal Control DNA shall be stored at -20°C in appropriate aliquots. It is also possible to store aliquots of the master mix ready-to-use in PCR-tubes at -20°C.
- Make sure not to contaminate the Internal Control DNA during reconstitution, aliquoting or pipetting in general. To guarantee highest sensitivity of mycoplasma detection, we adjusted precisely the lowest possible concentration of the competing Internal Control DNA – which makes it very susceptible to microbial degradation.
- Use an appropriate DNA polymerase. The reaction mixture is optimized for Hotstart Taq DNA Polymerases; we recommend SuperHot Taq DNA Polymerase (A5231)
- Use sterile, DNA-free tubes. Caution: Do not autoclave your PCR tubes if the autoclave is used for sterilization of (microbial) waste as well. Autoclaves are a source of nucleic acid contaminations in laboratories and poorly suited to destroy nucleic acids. During autoclaving, fragmented DNA molecules are released in large quantities with the steam, easily contaminating materials and surfaces that came in contact with.
- Samples should be derived from the supernatant of cultures that are at 90-100 % confluence. With average titers at 106 and a maximum titer at 108, sufficient amounts of mycoplasma DNA are present to guarantee a sensitive PCR.
- Heat-inactivate the sample material. Sample material derived from cell cultures contains DNases which can degrade mycoplasma DNA even at low temperatures. Therefore, we recommend heat-inactivation (direct heating of the cell culture supernatant or the biological sample material) immediately after sampling - or DNA extraction.
- Make sure your sample does not contain any PCR inhibitors such as cell debris, alcohol or PBS buffer. PCR inhibiting substances may also accumulate in the medium of older cultures (>100% confluency).
- For critical sample materials, such as old cell cultures, cell pellets as well as Fetal Calf Serum and vaccines, a DNA extraction is strictly recommended prior to testing. Make sure to remove any alcohol containing wash buffer from the preparation to avoid co-elution of alcohol and sample material.
Troubleshooting

No Internal Control signal in the Positive Control (or a strongly contaminated sample).
In fact, this is not a problem, but very likely since both PCR reactions are competing! The Internal Control DNA is applied to demonstrate PCR inhibition in a mycoplasma-negative sample. If no mycoplasma DNA template is present in the reaction mixture, the Internal Control DNA is amplified exclusively. In the presence of mycoplasma DNA, the Internal Control DNA is strongly diluted, resulting in a very weak or even missing signal.

No Internal Control signal in the Negative Control (and in an apparently negative samples).
There are two possible reasons for this result: First, the Internal Control DNA was not added to the master mix or, second, it has become contaminated with bacteria, e.g. by a non-sterile pipette tip. Since the Internal Control DNA is characterized by a very low concentration - to guarantee the highest possible sensitivity of mycoplasma detection - it is rapidly degraded by bacteria.

No signal in the samples, neither for mycoplasma DNA, nor Internal Control DNA.
PCR inhibition. Reasons might be cell released metabolites that inhibit the DNA polymerase or other interfering substances like alcohol or phosphate containing buffers. Another possibility is that the sample material (cell culture supernatant!) may be contaminated by residual cell debris. In all these cases, a DNA extraction prior to PCR is required.
Treatment of contaminated cell cultures
How eliminating mycoplasma from an infected cell culture?
The easiest way to eliminate mycoplasma is to autoclave the contaminated cells together with any bottle of medium and solution used with this relevant culture. Don’t forget subsequent cleaning and disinfection of surfaces, hoods, incubators, pipettors etc. - or better, the whole lab! Make sure that other cell lines are not infected as well!
Since efforts and costs are both high, cleaning up a contaminated culture by anti-mycoplasma treatment only makes sense in case of very valuable or irreplaceable cultures – and if the potential source of mycoplasma is banished from the laboratory.
Despite assured resistances, the most reliable and efficient treatment of mycoplasma contaminations is the addition of suitable antibiotics, such as quinolones, tetracyclines and macrolides.
Besides the traditional mycoplasma-eliminating agents Myco-1 & 2 (tiamulin and minocyclin) and Myco-3 (ciprofloxacin), ITW Reagents offers a solution for effective and permanent removal of mycoplasma species from cell culture: Myco-4 provides a broad spectrum of activity (including any type of mycoplasma, acholeplasma, spiroplasma and entomoplasma) combined with very low cytotoxicity and a low resistance risk due to an initial biophysical mode of action.
| Prod. No. | Description | Features |
| A5240 | Myco-3 | Single-phase treatment with Ciprofloxacin. |
| A8366 | Myco-4 | 2-step treatment with a mycoplasma-specific biophysical reagent followed by an appropriate antibiotic combination. |

Mycoplasma Elimination by Myco-4: How does it work?!
Myco-4 is used for the elimination of mycoplasma, acholeplasma, spiroplasma, and entomoplasma in all kinds of cell cultures.
Myco-4 is a highly efficient combination of standard antibiotics and a biological agent. The biological agent integrates into the mycoplasma membrane and compromises its integrity. By combination with standard antibiotics, the effective dose of both, biological agent and antibiotics, can be reduced to a minimum for lowest cytotoxicity, still causing a highly reliable and definite elimination of mycoplasma. In addition, the biophysical properties of Myco-4 make the development of resistant strains very unlikely.
One application includes 4 vials, a Starter Treatment (red cap) and three Main Treatment (yellow cap) solutions. The Starter Treatment kills most of the mycoplasma particles without harming the cells. The Main Treatment kills the remaining particles, leading to a permanent eradication with efficiency rates up to 100%. Each component is a sterile, ready-to-use solution.
Contaminated cells are incubated once with the Starter Treatment solution (for the common time period of a normal passage, or at least 30 min) and three times with the Myco-4 Main Treatment reagent (each time for the time period of a passage).
Precondition for successful mycoplasma elimination is a limited number of single cells and a reduced concentration of serum during incubation with the Starter Treatment solution!
Key Factors
- Use a maximum of 105 cells for a treatment to keep low the mycoplasma load and to avoid interactions with the cell surface.
- Ensure that Myco-4 Starter Treatment is already present in the culture medium before adding cells. Add cells directly to the elimination mix to avoid evaporation (insert the pipette tip directly into the mix!).
- Since the Myco-4 Starter Treatment works by biophysical means through association with the mycoplasma membrane, the reagent needs direct contact with the mycoplasma particle in order to be effective. Do not treat cell clusters. We suggest using Trypsin (A3964) to detach the cells from each other and to smooth cell surfaces.
- Ensure treatment of single cells (check under microscope). If necessary increase duration of Trypsin treatment or detach the cells from each other by pipetting up and down.
- Mycoplasma elimination in highly concentrated solutions of proteins or lipids is not possible. The mycoplasmacidal activity of Myco-4 Starter Treatment is affected by lipids and proteins in the reaction mixture, e.g. components in supplemented fetal calf serum. These ingredients competitively bind Myco-4 Starter Treatment and prevent its binding to the mycoplasma membrane. Our protocol is applicable for a maximum of 5 % v/v of fetal calf serum. For the Myco-4 Main Treatment serum reduction to 5 % is no longer required; the FCS concentration can be adjusted to the usual content.
- Myco-4 is suitable for adherent as well as suspension cell lines. Cell lines that were already treated successfully include Vero, BHK21, GBK, ML, Hep2, 293, CRFK, Jurkat, Molt 4, MT-4. Viruses shall be treated in combination with their host cells. Cells harboring Chlamydia or other bacteria as host systems cannot be treated. The antibiotic portion of the product might affect the integrated microorganisms.
- Antibiotics, especially if required for selection, do not interfere with the elimination procedure. In rare cases cytotoxicity might be increased by unpredictable interactions of the reagents.
- Myco-4 does not penetrate the cellular membrane. Therefore it cannot eliminate intracellular contamination. However, mycoplasma is an extracellular contaminant. Mycoplasma penetrans is the only species described to persist intracellular. So far, M. penetrans has not been reported as contaminant in cell culture.
Mycoplasma Prevention

How can I avoid contaminations?!
Probably, there will never be a point in time when mycoplasma contaminations are completely banished from our labs – as long as humans are working there. But carrying out some general principles will surely minimize the risk of contaminations and prevent costly or embarrassing situations.
- Strictly follow aseptic techniques and practices, including no unnecessary talking, no mouth pipetting, no media supply by pouring, regular hand washing and disinfection! Do not use the laminary flow for storage of solutions and equipment! Only work with ONE cell line at a time and use separate materials for each cell line to avoid cross-contaminations! Make sure all media, solutions and materials are properly sterilized – the same holds true for any kind of occurring waste of course!
- Frequently clean and disinfect surfaces, laminary flows, incubators, water baths and all other equipment – before AND after the working procedure. Make sure the laboratory is cleaned up regularly and only authorized persons have access to the working area.
- Use antibiotics responsibly. For routine culture work antibiotic-free media should be employed. General usage of antibiotics to mask low hygiene levels, lack of good aseptic techniques or improper cell culture facilities is no remedy! Quite the contrary, non-responsible use of antibiotics will make the situation even worse. Especially mycoplasma contamination rates are found to be much higher in cell lines grown in antibiotic-containing medium than in antibiotic-free cultures. Most common antibiotics used in cell culture do not act on mycoplasma! Besides generally ineffective beta-lactams, very high resistance levels of mycoplasma against streptomycin, kanamycin, gentamicin, and neomycin were determined.
Anyhow, there exist useful applications for antibiotics in cell culture, e.g. within the first two weeks of primary culture. In order not to create new resistances due to inactivation of the antibiotic, the antibiotic-containing medium should be refreshed frequently. As an excellent alternative to classical cell culture antibiotics like PenStrep, ITW Reagents provides a product to prevent microbial growth in cell cultures: CellCultureGuard A8906. This combination of selected antibiotics (one being a fluoroquinolone) offers a wide range of anti-microbial activity, making it our first choice cell culture reagent: CellCultureGuard is active against extra- and intracellular bacteria, mycoplasma, protozoa and fungi (yeast). Additionally, it is highly compatible with resistance markers and bears a low risk of resistance development. - Isolate incoming cell cultures (use a separate incubator or at least sealed flasks as well as separate culture media and materials) until the mycoplasma test results are proven to be negative.
- Test frequently for contamination – regardless of whether the cell culture contains any antibiotics or not! Routine testings for the presence of mycoplasma species are an absolute must for the responsible scientist! Only by identification and treatment or elimination of the infected cell line the risk of further (cross-) contaminations is banished and experiments yield stable and reliable results.
ITW Reagents offers a series of products preventing microbial contaminations - in hoods, incubators, water baths and cell cultures.
| Prod. No. | Description | Features |
| A5230 | Incubator-Clean™ | Non-toxic and biodegradable disinfectant for incubators and sterile benches; prevents contamination with and growth of fungi, bacteria (including mycoplasma) and viruses (including HIV and Hepatits B). Fully compatible with common work surfaces (non corrosive!) |
| A5219 | Incuwater-Clean™ | Non-toxic, non-volatile, and extremely effective disinfectant for CO2-incubator water baths. |
| A9390 | Aquabator-Clean (100X) | Disinfectant for prevention of microbial growth in common water baths. |
| A8906 | CellCultureGuard | Combination of especially selected antibiotics to prevent microbial growth (extra- and intracellular bacteria, mycoplasma, protozoa and fungi) in cell cultures; provides high compatibility with resistance markers and low risk of resistance development. |
The Mycoplasma-Story: What is important to know?
Firstly reported in 1956 (Robinson et al. 1956), the potential presence of mycoplasma in cell culture laboratories challenges scientists. The parasitic mycoplasmas represent a serious problem for all cell line-related fields in research as well as in industrial facilities for development or manufacture of cell-derived biological and pharmaceutical products, including vaccines, monoclonal antibodies, drugs, and products for gene and cell therapy. Still, there is no perceivable reduction of cell cultures infection rates (Ryan 2008), even though risks and consequences caused by mycoplasma infections are known for decades, and strategies for their prevention, detection and elimination are well established. Why are so many cell lines – while commonly well fostered by their cell culturists – still insufficiently protected against the cell wall-free invader? Is this a cause of carelessness, or rather a lack of knowledge? Unfortunately we cannot provide any data regarding this question – but a lot of facts demonstrating the importance of the unpopular subject.
How do mycoplasmas commonly enter our labs and cultures?
Mycoplasmas are omnipresent; their broad range of hosts includes humans and other mammals, birds, reptiles, fish, insects and plants (Razin et al. 1998). However, in cell culture laboratories, 95% of all continuous cell line infections are caused by only six species originally from bovine (M. arginini & Acholeplasma laidlawii), swine (M. hyorhinis) - and human (M. orale, M. fermentans, M. hominis) (Drexler and Uphoff, 2002). The main source of mycoplasma contaminations today are other, previously mycoplasma-infected cell cultures used in the same laboratory (Rottem & Barile 1993, Drexler et al. 2002; Drexler & Uphoff 2002). The infection may be transferred by aerosols, particulates and inadequate cell culture technique directly - or indirectly via media, solutions and laboratory equipment contaminated by previous use in processing mycoplasma-infected cells. As a result, 15-35% of all continuous cell lines are positive for mycoplasma, but only 1% of the primary cell cultures (Drexler & Uphoff, 2002). The second leading source is the laboratory personnel, explaining the fact that mycoplasma species from human are the most common contaminates (responsible for 40-80% of the infections) with M. orale, commonly colonizing the oral cavity, representing the primary species isolated from contaminated cell cultures.
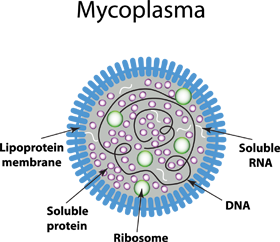
Mycoplasma species from bovine or swine can be traced back to contaminated sera and other animal-originating products, e.g. the prevalent presence of A. laidlawii and M. arginini implicates fetal or newborn bovine serum as the primary source of infection.
By now, sera and media are rarely the source of mycoplasma contamination (Lincoln & Lundin 1990; Armstrong et al. 2009) as long as they are purchased from reputable manufacturers that sterilize their products by several filtration steps using a 0.1 µm pore membrane filter and frequently control sterility.
What makes mycoplasma species worse than other bacterial contaminates – and why is it a must to banish them from cell cultures?
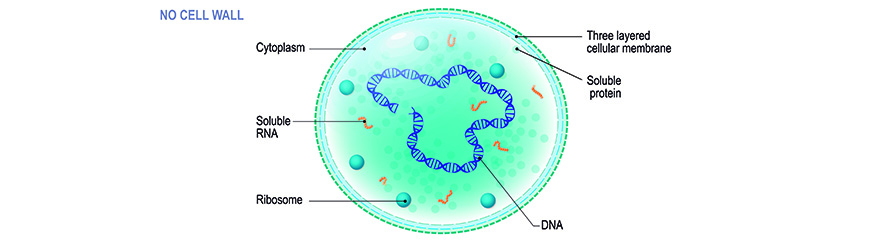
In contrast to “common” bacteria, these tiny prokaryotes do not possess a cell wall. Together with other cell wall-lacking bacteria - species of ureaplasma, acholeplasma, anaeroplasma, spiroplasma - they form the class of mollicutes. Nevertheless, the terms “mycoplasma” or formerly “pleuropneumonia-like organisms (PPLO)” and “mollicutes” are often used synonymously. Due to the absence of a cell wall, mycoplasmas are unaffected by antibiotics that interfere with peptidoglycan formation, namely beta-lactam antibiotics. These include penicillin-derivatives, cephalosporins, and carbapenemes. Furthermore they are very flexible in shape which in addition to their small size (ranging from 0.1 to 0.8 µm in diameter, depending on the literature) makes them difficult to filter from solutions. Mycoplasma species easily penetrate the membrane of 0.2 µm filters commonly used for sterilization of media, sera and other in-autoclavable reagents. Mycoplasma’s general dependence on complex enriched media (including host cell nutrients) and defined environmental conditions – both perfectly realized in cell culture – and their very slow growth rates complicate identification of infected cells by common microbiological cultivation methods. Their small size and missing cell wall allows them to achieve high densities in cell cultures; often without being detectable by turbidity, cytopathogenicity or even microscopic examination. However, the consequences of mycoplasma contaminations should not be underestimated; neither in regard to research (and the researcher’s career!), nor in terms of serious health risks for humans and animals.
Please keep in mind that the mycoplasma family is composed of a number of pathogenic organisms!
By growing covertly and undisturbed within a cell culture, mycoplasma can easily take over the control of reagents, equipment, and other cell lines within weeks (McGarrity 1976). Be aware that the lack of visible effects provides a false sense of security: While often behaving inconspicuous at first glance, the fastidious organisms are able to influence nearly every single cellular function, ranging from a decelerated growth rate to metabolic (including protein, RNA, DNA synthesis) and morphologic changes. All these effects are mainly based on a competition for essential nutrients (nucleosides, nucleotides, nucleobases, arginine and other amino acids, fatty acids, sugars, etc.) and the release of toxic, cytolytic or acidic metabolites. By up- and down-regulation of cytokines and growth factors, stress-response genes, transport proteins, receptors, ion channels, oxidases, tumor suppressors and oncogenes, mycoplasmas significantly alter the gene expression profiles of cultured cells (Miller et al. 2003). Therefore they make any experiment carried out with infected cells questionable! Furthermore they are known to cause chromosomal aberrations in vitro, with chromosomal breakage, translocation events, and reduction or augmentation in chromosome number being the most frequent outcomes. Virus propagation might also be influenced in both directions, positively (by inhibiting interferon induction and activity) as well as negatively (by competing for essential nutrients). Even though there are a large number of potential effects described in literature, it is unpredictable which effect will occur. Possible effects depend on mycoplasma species and strain, the infected cell type, and certainly on environmental conditions (Rottem & Barile 1993).

Finally, besides biosafety concerns, the consequences of mycoplasma contamination on laboratory work are loss of time, efforts, money (regarding cells, media, materials, but also valuable biopharmaceuticals if cultures were used for production of vaccines, antibodies or drugs) and good reputation. Research based on mycoplasma-contaminated cell lines will produce inaccurate or erroneous results yielding misleading publications. Consider the personal embarrassment and maybe the loss of good reputation if the published results are proven to be faulty due to a contamination problem. And how awkward will it be to get informed by a colleague that the cell line you provided him is contaminated? All these factors should be rethought when calculating to take the risk of covert mycoplasma infections by NOT testing cell cultures and NOT actively preventing them by good laboratory practise.
To sum up, a mycoplasma-free cell culture is PRECONDITION for safety and purity of cell-derived products and reliable results in scientific experiments.
The good message: it is possible to minimize the risk of general mycoplasma contaminations – and to exclude serious outbreaks.
The “mycoplasma problem” is known for decades - why does it still exist?!
There are two main reasons why mycoplasma contaminations are not banished from cell culture laboratories yet: First, half of the researchers still do not test their cell cultures for mycoplasma (Ryan 2008) and second, there is a tendency to rely on antibiotics instead on good aseptic practices.
Even though cell culture experts agree that general use of antibiotics can increase the severity of contamination problems, the routine use of antibiotics in cell culture laboratories is still prevalent. Particularly mycoplasma contamination rates are much higher in cell lines grown in antibiotic-containing medium than in antibiotic-free cultures (Barile 1973). If microorganisms, bacteria or fungi, are accidentally brought into antibiotic-free culture medium, they will replicate non-inhibited, soon leading to visible indicators of contamination: turbidity, filamentary structures, color changes due to pH alteration. In contrast, the presence of antibiotics will prevent the microbial growth – maybe. Unfortunately there is no absolute guarantee that the added antibiotics act against the introduced microorganisms (probably a mixture of different species), and sooner or later the user will face some kind of resistance phenomenon. If the introduced germ is fully resistant to the antibiotic, it will hopefully rapidly overgrow the culture and become visible within a short period of time. If the introduced microorganism only shows a partial resistance the situation is worse. Due to the latent static level of partly resistant contaminations, the risk of cross contaminations and usage of the affected culture in experiments or bio-production should not be underestimated. This worst case is very likely if the invader belongs to the species of mycoplasma (e.g. brought into the culture through aerosol droplets from the mouth of the cell culturist), since most common antibiotics used in cell culture do not act on mycoplasma! Besides the beta-lactams being ineffective anyway, high resistance levels of mycoplasma against streptomycin (88%), kanamycin (73%), gentamicin (80%) and neomycin (86%) (Lundin & Lincoln 1994) were determined.
Apart from Barile's observation of strongly increased rates of mycoplasma contamination, morphological and functional changes are other disadvantages one has to take into account (Kuhlmann 1996) when using antibiotics on a routine basis. Anyhow, there exist useful applications for antibiotics in cell culture, e.g. within the first two weeks of primary culture. In order not to create new resistances due to inactivation of the antibiotic, the antibiotic-containing medium should be refreshed frequently.
References
- Armstrong SE, Mariano JA and Lundin DJ (2010) The Scope of Mycoplasma Contamination within the Biopharmaceutical Industry. Biologicals 38: 211-213.
- Barile MF, Hopps HE, Grabowski MW, Riggs DB and Del Giudice RA (1973) The Identification and Sources of Mycoplasmas Isolated from Contaminated Cell Cultures. Ann. NY Acad. Sci. 225:251-264
- Drexler HG and Uphoff CC (2002) Mycoplasma Contamination of Cell Cultures: Incidence, Sources, Effects, Detection, Elimination, Prevention. Cytotechnology 39:75-90.
- Drexler HG, Uphoff CC, DirksWG and MacLeod RAF (2002) Mixups and mycoplasma: The enemies within. Leukemia Res 26:329–333.
- Kuhlmann I (1996) The Prophylactic Use of Antibiotics in Cell Culture. Cytotechnology 19: 95-105.
- Lincoln CK and Lundin DJ (1990) Mycoplasma Detection and Control. United States Federation for Culture Collection Newsletter 20 (4):1-3.
- Lundin DJ and Lincoln CK (1994) Mycoplasmal Contamination of Cell Cultures within the Clinical Diagnostic Laboratory. Amer. Clin. Lab. April (4):6
- McGarrity GJ (1976) Spread and Control of Mycoplasmal Infection of Cell Cultures. In Vitro 12:643-647.
- Miller CJ, Kassem HS, Pepper SD, Hey Y, Ward TH, Margison GP (2003) Mycoplasma infection significantly alters microarray gene expression profiles. BioTechniques 35:812-814.
- Razin S, Yogev D and Naot Y (1998) Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev 62, 1094-1156.
- Robinson LB, Wichelhausen RH and Roizman B (1956) Contamination of Human Cell Cultures by Pleuropneumonia-like Organisms. Science 124:1147-1148.
- Rottem S and Barile MF (1993) Beware of Mycoplasmas. Trends in Biotechnology 11:143-150.
- Ryan JA (2008) Understanding and Managing Cell Culture Contamination. Corning, Inc. Technical Bulletin
